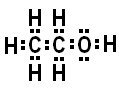Period G
Mrs. Fontaine
Molecular Formula: C2H6O
Key:
Grey= Carbon (C)
White= Hydrogen (H)
Red= Oxygen (O)
This second picture represents electronegativity of the atoms of the Ethanol molecule. The arrows starts at the less electronegative atom, and points to the atom with a higher electronegativity value.
Example: Hydrogen has an electronegativity value of 2.1
Carbon has an electronegativity value of 2.5
Thus the arrow must start at Hydrogen with a lower value, and point towards carbon with the higher value.
What is Ethanol exactly?
Ethanol, otherwise known as Ethyl alcohol or grain alcohol, is an alcohol-based substitute fuel for unblended gasoline. When this said Ethanol is blended with gasoline, it provides a higher octane rating, and releases fewer toxic emissions.
Ethanol is also the primary ingredient in alcoholic drinks and serves as a depressant to the human mind. It is absorbed in the gastrointestinal tract and reacts violently with oxidants.
*Mostly made from corn in the U.S
Bond Nature
In an Ethanol molecule, we have three types of bonds, lets look at them.
Carbon (C) 2.5 - Hydrogen (H) 2.1 = .4
.4 is extremely polar covalent.
Oxygen (O) 3.5 - Carbon (C) 2.5 = 1
1 is moderately polar covalent.
Oxygen (O) 3.5 - Hydrogen (H) 2.1 = 1.5
1.5 is slightly polar covalent.
Polar or Non- Polar?
Ethanol (C2H6O) is for sure a polar molecule.
Lets look here.
This molecule would be non- polar if the oxygen was non-existent. But if it wasn't, it wouldn't be Ethanol! The two unshared electron pairs of oxygen is the most negative part of the molecule, but it is not present on the other side of the molecule, therefore, the molecule is polar because the unshared pairs cause an extreme imbalance throughout the molecule.
Intermolecular Forces
London Dispersion
The London dispersion force is the weakest intermolecular force. The London dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. This force is sometimes called an induced dipole-induced dipole attraction. London forces are the attractive forces that cause non-polar substances to condense to liquids and to freeze into solids when the temperature is lowered sufficiently. (Perdue Science)
- This force of attraction is found in every molecule, both non-polar and polar.
Dipole- Dipole
Dipole-dipole forces are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule. (Perdue Science)
- This force of attraction is found only in polar molecules.
Hydrogen Bonding
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, like nitrogen, oxygen or fluorine.
- Hydrogen Bonding occurs only in molecules that hydrogen can bond with Nitrogen (N), Oxygen (O), and Fluorine (Fl) of another molecule.
In Ethanol, we have cases of all three intermolecular forces.
- We have London dispersion present because it is a polar molecule, and the electrons can form temporary dipoles with other molecules.
- We have Dipole- dipole present because it is a polar molecule, and because Ethanol can attract from positive and negative ends of the molecules.
- We also have the least common force, Hydrogen bonding present. With all of the Hydrogens on the outside of the molecule, Ethanol has many opportunities to bond with the oxygen, nitrogen, or fluorine of other molecules.
Make the Switch!
Are you tired of spending 3+ dollars at the pump for gasoline derived from fossil fuels? If so, make the switch !
In a recent study, it was found that making a gallon of ethanol requires 95% less petroleum than a gallon derived from fossil fuels! 95%!
This means that we would have 95% less greenhouse gases clogging up our atmosphere!
So make the switch today, to better the planet we live on.
Think of it this way, if you made the switch, you would make your car a whole lot happier, as well as your wallet!