Period G
Mrs. Fontaine
Molecular Formula: C2H6O
Key:
Grey= Carbon (C)
White= Hydrogen (H)
Red= Oxygen (O)
This second picture represents electronegativity of the atoms of the Ethanol molecule. The arrows starts at the less electronegative atom, and points to the atom with a higher electronegativity value.
Example: Hydrogen has an electronegativity value of 2.1
Carbon has an electronegativity value of 2.5
Thus the arrow must start at Hydrogen with a lower value, and point towards carbon with the higher value.
What is Ethanol exactly?
Ethanol, otherwise known as Ethyl alcohol or grain alcohol, is an alcohol-based substitute fuel for unblended gasoline. When this said Ethanol is blended with gasoline, it provides a higher octane rating, and releases fewer toxic emissions.
Ethanol is also the primary ingredient in alcoholic drinks and serves as a depressant to the human mind. It is absorbed in the gastrointestinal tract and reacts violently with oxidants.
*Mostly made from corn in the U.S
Bond Nature
In an Ethanol molecule, we have three types of bonds, lets look at them.
Carbon (C) 2.5 - Hydrogen (H) 2.1 = .4
.4 is extremely polar covalent.
Oxygen (O) 3.5 - Carbon (C) 2.5 = 1
1 is moderately polar covalent.
Oxygen (O) 3.5 - Hydrogen (H) 2.1 = 1.5
1.5 is slightly polar covalent.
Polar or Non- Polar?
Ethanol (C2H6O) is for sure a polar molecule.
Lets look here.
This molecule would be non- polar if the oxygen was non-existent. But if it wasn't, it wouldn't be Ethanol! The two unshared electron pairs of oxygen is the most negative part of the molecule, but it is not present on the other side of the molecule, therefore, the molecule is polar because the unshared pairs cause an extreme imbalance throughout the molecule.
Intermolecular Forces
London Dispersion
The London dispersion force is the weakest intermolecular force. The London dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. This force is sometimes called an induced dipole-induced dipole attraction. London forces are the attractive forces that cause non-polar substances to condense to liquids and to freeze into solids when the temperature is lowered sufficiently. (Perdue Science)
- This force of attraction is found in every molecule, both non-polar and polar.
Dipole- Dipole
Dipole-dipole forces are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule. (Perdue Science)
- This force of attraction is found only in polar molecules.
Hydrogen Bonding
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, like nitrogen, oxygen or fluorine.
- Hydrogen Bonding occurs only in molecules that hydrogen can bond with Nitrogen (N), Oxygen (O), and Fluorine (Fl) of another molecule.
In Ethanol, we have cases of all three intermolecular forces.
- We have London dispersion present because it is a polar molecule, and the electrons can form temporary dipoles with other molecules.
- We have Dipole- dipole present because it is a polar molecule, and because Ethanol can attract from positive and negative ends of the molecules.
- We also have the least common force, Hydrogen bonding present. With all of the Hydrogens on the outside of the molecule, Ethanol has many opportunities to bond with the oxygen, nitrogen, or fluorine of other molecules.
Make the Switch!
Are you tired of spending 3+ dollars at the pump for gasoline derived from fossil fuels? If so, make the switch !
In a recent study, it was found that making a gallon of ethanol requires 95% less petroleum than a gallon derived from fossil fuels! 95%!
This means that we would have 95% less greenhouse gases clogging up our atmosphere!
So make the switch today, to better the planet we live on.
Think of it this way, if you made the switch, you would make your car a whole lot happier, as well as your wallet!


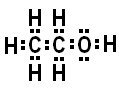


Overall appearance: I liked the color coordination of each section to divide the topics; this made it easier to focus on each point separately. The background is also appropriate, and the black background for the wording is sleek :)
ReplyDeletePicture: The explanation of why the arrows are pointing from hydrogen to carbon is clear and accurate, but the only material left unsaid is the electronegativity charge of the oxygen and why the carbon arrow is pointing to the oxygen.
Polarity: To my knowledge, it is accurate to say that ethyl alcohol is polar.
Intermolecular Forces: The definitions are clear and easy to understand, and I found it helpful that bullet points were included after the definitions stating the requirements of the nature of the molecule in order for it to apply.
Ad: I found your ad convincing, except something important to mention in selling a form of fuel would be the safety precautions of it. Does it have hazardous components that could cause issues? I do like the cartoon picture at the bottom, and the price reference is persuasive.
This comment has been removed by the author.
ReplyDeleteI really like the overall appearance of the blog, and I think that the changes in colors mix things up and make the text eye catching. I like the use of the comic and the use of more pictures than required.
ReplyDeleteThe drawing is accurate, as are the arrows indicating polarity. The key made it easy to understand, and the picture looked nice even with the arrows all over it.
The molecule was correctly identified as a polar, and the bonds were correctly identified as polar covalent in nature.
The intermolecular forces were accurate. I liked the way that they were presented with the definitions first, and then which forces were present in the molecule.
I felt the ad was convincing, and it showed the benefits of switching to ethanol well. I enjoyed the cartoon, and also the line about making your car happier.
The overall appearance is good, the colors & texts work well together. It was a good idea to change the colors of key words.
ReplyDeleteThe picture was good, the use of arrows & the key made it easy to understand.
This is accurately identified as a polar molecule.
The intermolecular forces are correct. The definitions made it easy to understand.
The ad is convincing, especially with the enviornment the way it is now, this is a very good ad.
Overall Appearance: The overall appearance of the blog is very nice. I love the background and the white font on the black background works very well. I also like how you mixed up some of the font colors.
ReplyDeleteDrawing: The picture was excellent! The key made the drawing very easy to understand. The arrows in the drawing correctly showed the polarity of the molecule. It was an easy picture to understand and it worked well with the rest of the blog.
Polarity: The molecule was correctly identified as polar. The arrows were correctly drawn as well.
Intermolecular forces: The intermolecular forces were accurately described and easy to follow. The definitions were easy to understand. You did a great job of typing them out and leaving space to talk about them.
Advertisement: I enjoyed the advertisement very much! I thought that is was convincing. It made we want to "make the switch" and start using ethanol. I also enjoyed the cartoon and the picture of the gas pump. Great job!